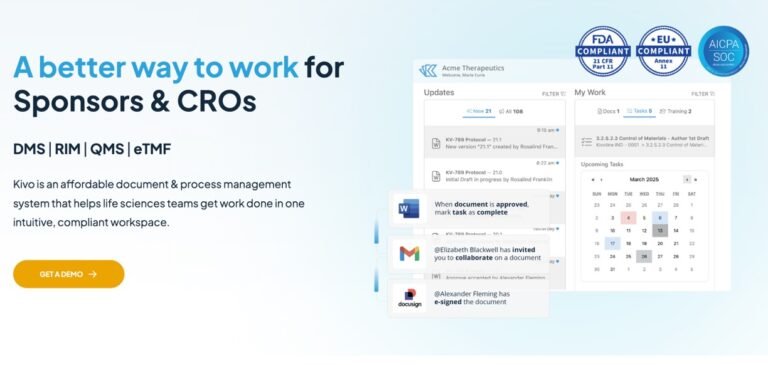
Kivo is a cloud-based document and process management system specially designed for scaling pharmaceutical and life sciences teams. It provides an intuitive, all-in-one workspace where teams can manage regulated documents, collaborate in real time, track compliance, and streamline submissions—without complex setup or steep learning curves. Kivo brings regulatory, quality, and clinical functions together, supporting everything from collaborative editing and electronic signatures to active trial management, ensuring your organization is always audit-ready and works efficiently.
Key Features:
-
Unified workspace for regulated document management, project tracking, and compliance workflows.
-
Collaborative editing in Microsoft Office with real-time updates and secure Part 11-compliant electronic signatures.
-
Customizable workflow automation including SOP authoring, approval, training, and audit tracking.
-
Advanced security with encryption, permission controls, and full audit logs for every document action.
Use Cases:
-
Manage all regulatory submission documents and timelines to stay compliant and audit-ready.
-
Streamline Standard Operating Procedure (SOP) creation, review, training, and version tracking.
-
Support ongoing clinical trials from study start-up to closeout with centralized trial master file (TMF) management.